 Photo: Getty Images
Photo: Getty Images
My kids are all about the latest technology: the coolest, fastest smartphones, video games, computers, gadgets of every kind. “What will they think of next?” is the way we traditionally marvel about it. And, as Americans, we celebrate it with our dollars. But when it comes to medicine, the latest is often not the greatest. There are risks when you are putting something new in your body and there can be consequences that are more significant than trying a new piece of electronics.
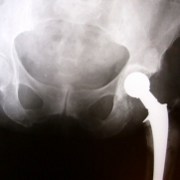
The latest example is hip implants. These are the medical devices used when someone has debilitating hip pain – usually from arthritis. A new hip ball and socket could give that person back their mobility and relieve them from pain. Hip joint replacement has been around for years and several companies make hundreds of millions of dollars selling them. It’s lucrative for orthopedic surgeons and hospitals too. It’s competitive. Should you have this model or that one? Should you go to Dr. Smith at hospital X where he has performed more with the new device than anyone in the region? Or should you go to Dr. Brown who is more conservative? Marketing muddies the water, and your interest in getting the latest medicine has to offer colors your choice.
If you decided to go with the newest and latest version of the hip replacement device, thinking that newer means better, in this case it appears that you may have picked wrong. It is turning out that fairly new, U.S. Food and Drug Administration-approved metal on metal hip joints have a problem that wasn’t seen in lab testing. Some of the time microscopic pieces of metal from the joints are floating around the body. And the doctors who pledged to “do no harm" unwittingly did. Devices are being removed in second surgeries.
As a patient you’d have to wonder, how did this happen? How did the FDA approve something that later proved harmful? Why did board certified doctors stake their reputations on something new that turned out to be inferior to, and more risky than, the old standby approach? Unfortunately, that is the state of medicine today. Companies are under pressure to come out with new models; the FDA is inclined to approve them if they are fairly similar to the old ones; and doctors and hospitals want to ballyhoo that they can provide a service that’s different from the competitor down the street.
But where does that leave you, the person in pain, or wanting to try a new medicine, or a new heart device or new medical gizmo?
Lately, there have been many instances in non-cancer health areas where, as more people received the device, or the pills, or the procedure, problems started showing up. It’s kind of like buying a new car when the model is brand new. Maybe the kinks need to be worked out. But when it comes to your health, the consequences of “kinks” are a much bigger deal.
As a cancer survivor, I know with some diseases we have a long way to go and experimental approaches in clinical trials or as just-approved therapies can be lifesaving. But in areas like joint replacement, where good options have existed, be careful. Don’t get caught up in the hype. It could save you from having a “revision” surgery and a lot more pain than you ever expected.
About the author: Andrew Schorr is a medical journalist, cancer survivor and founder of Patient Power, a one-of-a-kind company dedicated to bringing in-depth information to patients with cancer and chronic illness. Audio and video programs, as well as transcripts, help patients make informed decisions to support their health in partnership with their medical team. Patient Power is at www.PatientPower.info and on Facebook. Schorr is also the author of “The Web Savvy Patient: An Insider's Guide to Navigating the Internet When Facing Medical Crisis." http://www.websavvypatient.com/
Edited by Alison Stanton




Add a Comment1 Comments
The FDA will be quick to point out they do not 'approve' drugs or medical devices, the 'clear' them. Mr. Schorr is quite correct, though, there is no need for a drug or device to be an improvement on time tested items already on the market; they must only submit data proving equivalency in effectiveness and safety. Although testing --often requiring years--is done there is no way to expose the drug or device to the number of applications it will see in the real world; therefore, the lower probability risks will escape notice until after market release. A conservative physician will steer patients to the standard-of-care treatment and await further reports.
June 27, 2011 - 7:58amThis Comment